GMP Compliance in Weigh & Dispense Systems
By Punit Instrument Pvt. Ltd.
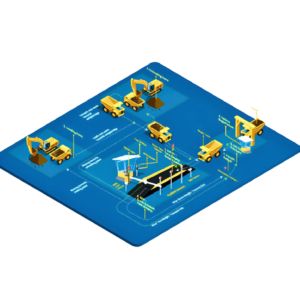
Weigh & Dispense Systems by Punit Instrument Pvt. Ltd. are designed to comply with Good Manufacturing Practices (GMP), ensuring accuracy, traceability, data integrity, and contamination control during material weighing and dispensing operations in regulated industries.
What is GMP in Weigh & Dispense?
GMP compliance in weighing and dispensing ensures that:
-
Correct material is weighed
-
Correct quantity is dispensed
-
All activities are documented, traceable, and auditable
-
Risks of mix-up, contamination, and human error are minimized
This is critical for pharmaceutical, biotechnology, food, and chemical manufacturing.
Key GMP Requirements Addressed
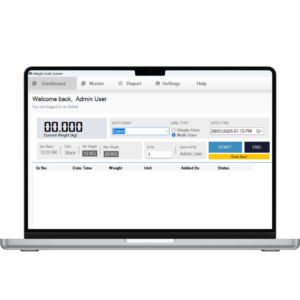
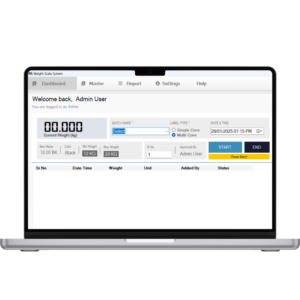
1. Accuracy & Precision
-
High-resolution load cells and weighing indicators
-
Stable weight reading with digital filtering
-
Regular calibration with NABL-traceable test weights
-
Target vs actual weight validation
2. Material Identification & Control
-
Material master with unique codes
-
Barcode / QR code-based material identification
-
Prevention of wrong material selection
-
Recipe & BOM-based dispensing
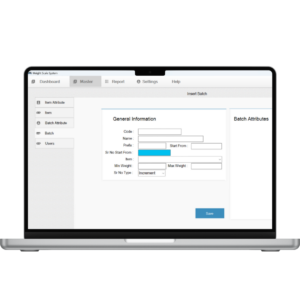
3. Batch & Lot Traceability
-
Batch / lot number tracking
-
Weighment linked to production batch
-
Complete history from raw material to finished product
-
Electronic batch records (EBR)
4. User Access & Control
-
Role-based user login (Operator / Supervisor / QA)
-
Password-protected access
-
Authorization for weight correction or override
-
Electronic signatures (optional)
5. Data Integrity (ALCOA Principles)
-
Attributable – operator ID recorded
-
Legible – clear digital records
-
Contemporaneous – real-time data capture
-
Original – source data stored securely
-
Accurate – validated weighing values
Audit trails are maintained for:
-
Logins
-
Weighing actions
-
Modifications & corrections
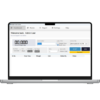
6. Documentation & Records
-
Automatic recording of:
-
Gross / tare / net weight
-
Date & time
-
Material code
-
Batch number
-
Operator ID
-
-
Secure storage of weighment data
-
Easy retrieval during audits
7. Labelling Compliance
-
GMP-compliant label printing after dispensing
-
Label includes:
-
Material name
-
Batch / lot number
-
Net weight
-
Status (Approved / Under Test / Rejected)
-
Date, time & operator
-
-
Barcode / QR code for traceability
8. Environmental & Equipment Compliance
-
SS construction (SS 304 / SS 316) where required
-
Smooth, easy-to-clean surfaces
-
Controlled access weighing areas
-
Compatible with cleanroom operations
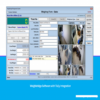
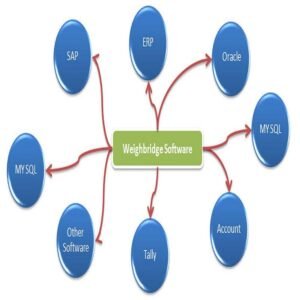
System Components (GMP-Oriented)
-
GMP-compliant weighing scales
-
Industrial weighing indicators
-
Weigh & dispense software
-
Barcode scanners & label printers
-
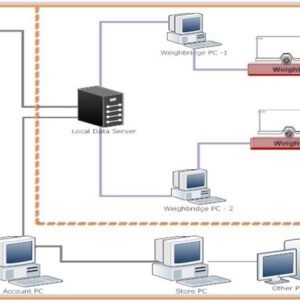
Secure database & server
Validation & Compliance Support
-
IQ (Installation Qualification)
-
OQ (Operational Qualification)
-
PQ (Performance Qualification)
-
SOP & documentation support
-
Calibration & revalidation support
Industries Served
-
Pharmaceutical manufacturing
-
API & formulation plants
-
Biotechnology units
-
Nutraceutical & food processing
-
Specialty chemical industries
Benefits of GMP-Compliant Weigh & Dispense
✔ Eliminates dispensing errors
✔ Ensures regulatory compliance
✔ Improves batch consistency
✔ Simplifies audits & inspections
✔ Enhances data integrity & security
Why Choose Punit Instrument Pvt. Ltd.?
Punit Instrument Pvt. Ltd. provides GMP-compliant weighing and dispensing solutions, including precision weighing hardware, validated software, labelling systems, ERP integration, and complete documentation support, ensuring smooth regulatory audits and reliable production operations.




Reviews
There are no reviews yet.